Future Food #2: Artificial photosynthesis
The benefits and possible foundations of a synthetic food supply
Modern agriculture is only 0.0125% efficient
In the last post, I highlighted the inefficiency of plants at turning solar energy into stored chemical energy that we can eat. Because the most efficient crops capture <1% of the solar energy that falls on a patch of ground, and because the total pool of energy drops off exponentially at higher trophic levels, it takes a huge land area to produce the human food supply.
While enough solar energy falls on 0.75 m2 of land to meet one person’s theoretical energy needs, we currently farm ~6,000 m2 of land to feed one person1. Crudely, this means that modern agriculture is about 0.0125% efficient at turning sunlight into food.
Why does land efficiency matter?
The problem with inefficient agriculture is only apparent when you zoom out.

Food security risks
The obvious problem is the amount of space that it already takes to produce food. We have space to feed a bigger population, but not a significantly bigger one. Looking at the picture above, we’d have difficulty doubling our food production capacity assuming current diets, productivity, and food waste levels.
A more pressing concern is that if one region becomes much less productive (e.g, due to warmer climates or a Dust Bowl), we don’t have a large buffer of land where new agriculture could come from2. Arable land could become more scarce in the future, or migrate across borders. By the way, this picture is fairly representative of the whole world, which has about half of all habitable land dedicated to agriculture.
Carbon opportunity costs
The less-obvious problem is that all agricultural land was once occupied by grasslands, forests, and other wild ecosystems. Sentimentality aside, we should care about natural ecosystems for purely selfish reasons: they sequester a lot of CO₂, much more than pasture or cropland.
One mind-blowing finding from Hayek et al. is that If we stopped using the land dedicated to livestock farming (i.e, letting native vegetation regrow), we could remove about 800 gigatons of CO₂. That’s about half of cumulative anthropogenic emissions since the industrial revolution3. Eisen & Brown plugged several livestock4 phaseout scenarios like this into a climate model, and found that the negative emissions would effectively halt global warming for 30 years. Arable land is a valuable and scalable carbon sink.
Economic opportunity costs
Casey Handmer fondly describes solar panels as “inert glass rectangles that, placed on the ground, print out wealth, at roughly 100x the rate of the best farmland”. Chris Goodall compared a hectare of solar to a hectare of wheat in the UK, finding that a farmer could conservatively generate ~15x more revenue if they switched from cereal crops to photovoltaics. In a land-constrained place like the UK, these sorts of tradeoffs really matter – outsourcing one hectare of food production in favor of domestic energy production would be paid back 5-33 times over in reduced natural gas usage.
Energy is a more versatile commodity than food, so it might make sense to prioritize energy independence over food independence. Indeed, I’ll discuss some pathways that can be used to turn electricity into food below.
Synthetic food could be 10x more land efficient
The inefficiency of agriculture suggests a massive opportunity for improvement.
While plants are <1% efficient at turning sun into food, modern solar panels are about ~10% efficient (in practice) at capturing solar energy as electricity! If we could convert electricity to food at >10% efficiency, we could outperform crops. The existing literature indicates that we can do much better than that. For example, this paper from Leger et al. found that photovoltaic driven microbes could produce 10x more protein and 2x more calories per unit area than soybeans!
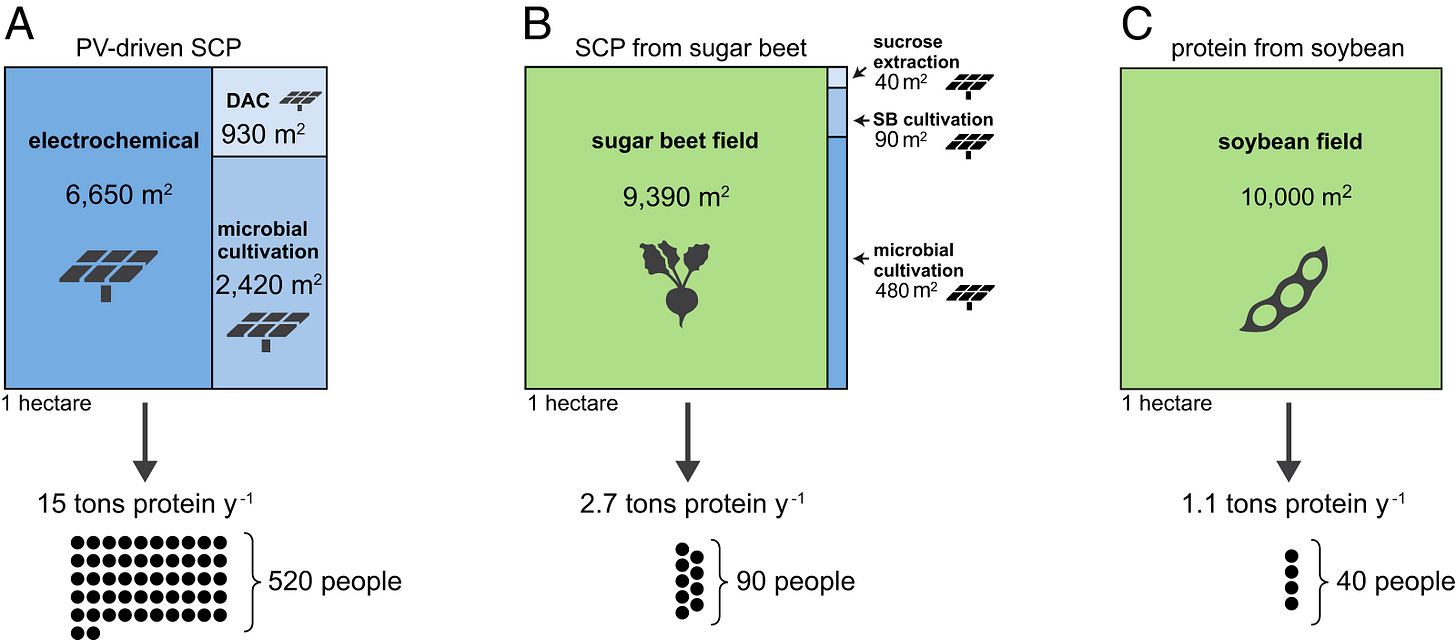
If our current agricultural efficiency is a mere 0.0125%, it should be possible to produce orders of magnitude more food in the same area, or the same amount of food using orders of magnitude less resources. The challenge is to find efficient and affordable ways to convert sunlight into molecules we can eat.
Primer: The solar industrial revolution
Several writers, especially Casey Handmer and Austin Vernon, have written extensively about an imminent solar-industrial revolution. It’s difficult to overstate the importance and impressiveness of solar energy, so I'll do it here briefly.
Solar is already the cheapest source of electricity on Earth, and is on track to get even cheaper (perhaps $10/MWh or 1 cent/kWh by 2030). Solar is a prototypical example of Wright's Law, meaning that the cost goes down by a predictable percentage (the learning rate) every time cumulative production doubles.
While the Wright’s Law implies that progress gets exponentially harder as you go, we're also increasing the rate of solar production exponentially, which nets out to a roughly linear looking cost decline each year. Even better, it appears that the learning rate for solar sped up around 2009, going from 27% to 44%.

Casey Handmer's essays have convinced me that for any commodity X, if we can commercialize a solar-to-X pathway, then X could become very cheap. His startup, Terraform Industries, is focused on synthetic hydrocarbons (e.g natural gas), but the broader solar industrial revolution could also give us cheaper chemicals, materials, fertilizers, carbon removal, manufacturing, compute power, and potentially, food.
There are several very important caveats.
First and foremost, cheap solar energy will only make your process cheaper if energy is a significant cost driver. If most of your cost-of-goods-sold (COGS) are CAPEX or labor, for example, cheaper energy doesn’t really help you.
Next, the solar-to-X process should ideally accept intermittent DC electricity as input, since that's what you get from a solar array. Just converting to AC adds significant cost (~30% more). Processes like electrolysis are well-suited for DC solar.
Adding batteries adds significant cost, and only makes sense if your process requires a high capacity factor to be economically viable (e.g due to high capital costs that must be recovered).
Lastly, just because solar is getting cheaper does not mean that grid electricity is getting cheaper. Energy prices from the grid will remain much higher due to transmission, distribution, losses, energy storage, profit margins, etc. So you need to co-locate your process with the solar.
So the cheapest solar-to-X processes will source their energy from onsite solar, accept DC electricity, and need to be economically viable under a low utilization factor (perhaps only 20-30% of the day).
The synthetic food revolution
The solar industrial revolution implies an agricultural revolution.
At lab scales, scientists and startups have already demonstrated processes for turning electricity into proteins, carbohydrates, and fats. This raises the possibility of a synthetic food supply that uses photovoltaics to bypass photosynthesis.
The back-of-the-napkin math suggests that artificial photosynthesis could be an order of magnitude more land efficient than biological photosynthesis. Furthermore, if solar cost curves prevail, synthetic foods may become the cheapest on Earth.
In the following sections, I’ll survey some of the most promising synthetic food pathways for proteins, carbohydrates, and fats.
Protein
Biomass fermentation using H2/CO2
A subset of autotrophs, called hydrogen oxidizing bacteria (HOBs), can grow off of an H₂/CO₂ gas mixture5. The hydrogen acts as an electron donor (providing energy), and CO₂ provides a carbon source. The CO₂ is assimilated directly through the Calvin-Benson-Bassham cycle, which is basically the same “dark reaction” that plants use for photosynthesis.

The easiest thing to make with HOBs is single-cell protein biomass, which is probably why Solar Foods, Air Protein, Farmless, and a few other companies are doing that first. Precision fermentation (manufacturing specific target proteins) is harder, and has not been commercially demonstrated using hydrogen to my knowledge. The EU's HYDROCOW project is attempting a demonstration.
My main concern around using hydrogen feedstock is intermittency and storage. Fed-batch or continuous fermentation requires a steady supply of feedstock, but cheap solar is only available for electrolysis during the day6. You could produce and store excess hydrogen during the day, but the consensus seems to be that hydrogen is expensive and difficult to store and transport.
Biomass fermentation using methane, methanol, or formate
More promising options, in my opinion, are single-carbon (C1) feedstocks like formate and methanol (liquid) or methane (gas). Several great papers enumerate the natural metabolic pathways by which autotrophs can consume these.
Today, most industrial fermentation processes use sugar, and genetic engineering efforts are needed to develop workhorse organisms that grow productively on C1 feedstocks. There are some notable exceptions like the Pichia pastoris yeast, which can grow on methanol. In fact, biomass fermentation on methanol using P. pastoris seems like the least technologically risky option of the ones I'm surveying.

Both methanol and formate are liquid at room temperature, making them much easier to store, transport, and work with than hydrogen. You can make renewable formate and methanol through the electrochemical reduction of CO₂, and methanol can also be made thermochemically. For this reason, I've seen both the “formate economy” and “methanol economy” proposed. They each have pros and cons; they’re both very soluble, but toxic to some organisms (and humans). Formate seems to support a wider range of organisms than methanol and produces less heat during fermentation, but methanol is more energy dense and would require less pH control.
We could build a formate or methanol economy, but why not leverage the methane economy we already have? Methane and methanotrophs might be an ideal combination because the feedstock is easy to store and transport, and we’ve built up substantial natural gas infrastructure already. If companies like Terraform Industries succeed, we’ll have a green methane supply that could be immediately plugged into the food system. Methane isn’t perfect though – it’s less soluble in water than methanol or formate which creates mass transfer limitations.
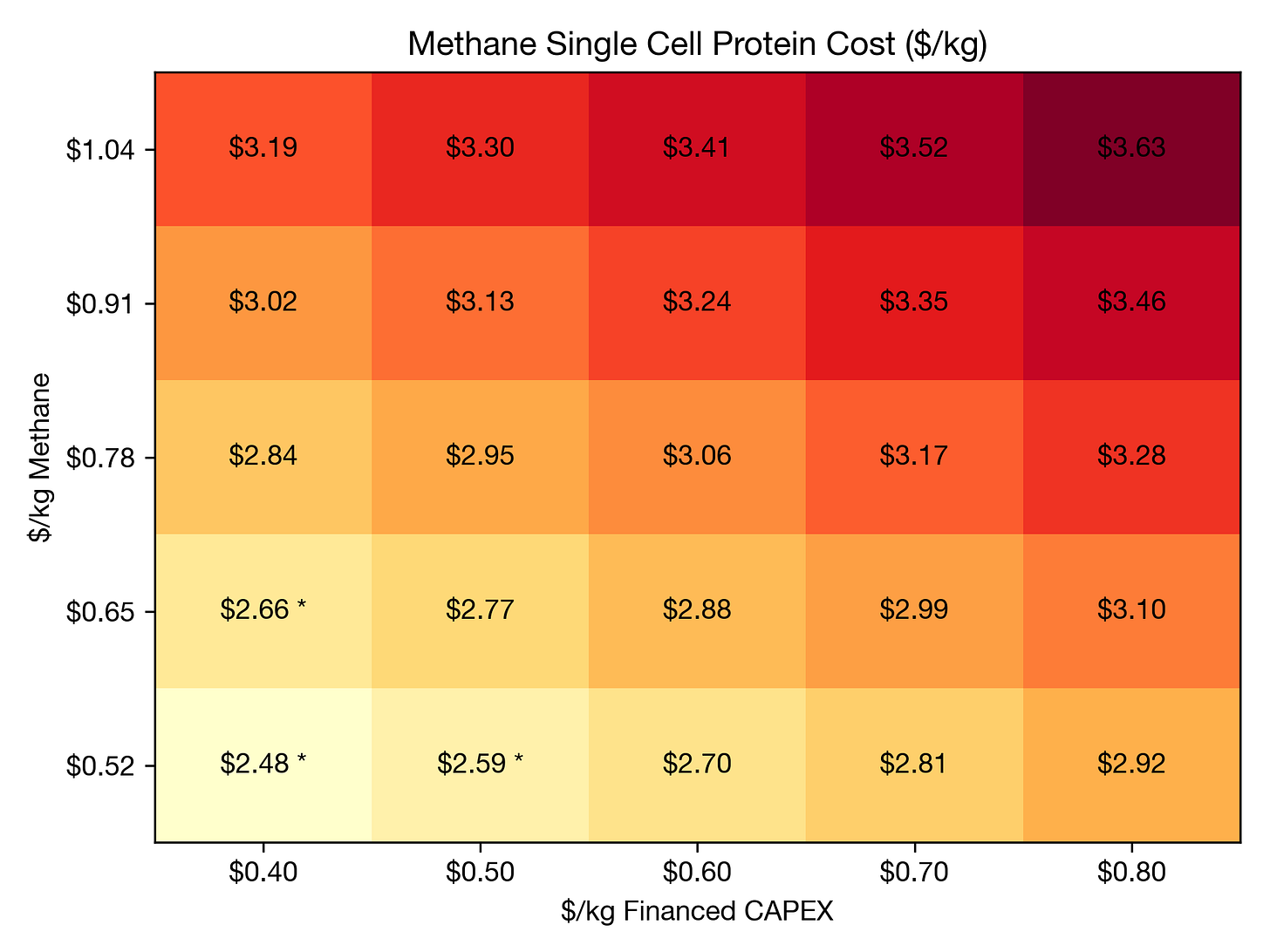
In the figure above, I’ve modeled the cost of methane single-cell protein (SCP) as a function of natural gas prices and facility CAPEX (including a WACC of 10%). The model is based on a hypothetical 100,000 metric ton per year facility and an organism that consumes 1.2 kg methane per kg of dry biomass yield. I’m also assuming other OPEX costs of $1.2/kg to account for electricity, water, waste, labor, etc, and a profit margin of 10%. My results are a bit more optimistic than those published elsewhere, which are in the $3-5/kg range.
My results suggest that if we can get green methane to price parity ($0.53/kg or ~$10/kcf) and reduce CAPEX, biomass fermentation can achieve price parity with soy protein isolate. This is an exciting but ambitious target. For reference, Terraform’s $10/kcf green methane target is based on subsystem targets of $0.89/kg H2 and $78/ton DAC CO2.
It’s also worth keeping in mind that soybean commodity prices fluctuate significantly, from as low as $0.20/kg to as high as $1.60/ton (a factor of 8). Soy protein isolate prices seem to be about ~5x higher than raw soybean prices, and my benchmark of $2.70/kg for soy protein isolate is based on a raw soybean price of ~$0.5/kg. So there is a potential future where soybeans get twice as expensive and biomass fermentation looks really favorable.
In regions with limited arable land, biomass fermentation could drastically improve food security. Single-cell protein is surprisingly versatile, and could become a staple ingredient in the food supply. However, I’m hesitant to draw sweeping conclusions about future of food from protein isolate prices. Though I’m an enthusiastic Huel drinker, I certainly wouldn’t be happy in a dystopian future where all of our protein comes in powered form!
Carbohydrates
Staple cereal crops, like maize, wheat, rice, and barley are the foundation of the human food system and supply most of our calories in the form of carbohydrates. Would be possible to set up an artificial carbohydrate supply that has all the aforementioned benefits of synthetic food?
Thermochemical Pathways (Formose Reaction)
The first possibility is to produce sugars thermochemically. This paper theorizes one possible route: from CO₂, to syngas, to methanol (C1), to formaldehyde (C3), to sugar (C6). Under assumed efficiencies for each step, the pathway would be 31% efficient at converting electricity into sugars. This paper enumerates a few other options. In both papers, the final step from formaldehyde to sugar (called the “formose reaction”) has a fatal flaw: it produces a racemic mixture of C1-C8 sugars, of which only the D-glucose (C6) enantiomer is edible. Despite its sweet taste, the L-glucose molecule cannot be metabolized by most organisms. I believe the formose reaction, and thermochemical pathways more generally, are a dead end because of their poor selectivity. Despite being discovered in 1959, I was unable to find an example of industrial sugar production using the formose reaction.

Chemoenzymatic Pathways
The second approach is to use an enzymatic process. Enzymes could introduce stereochemistry into the reaction pathway and improve the specificity of each step. Similar to cell-free protein synthesis, the idea behind cell-free carbohydrate synthesis is to carry out the steps of photosynthesis and gluconeogenesis outside of a cell. An end-to-end example is the ASAP cycle, whose authors produced starch in a cell-free process that can be fed with either methanol or hydrogen and CO₂. Many other cycles or linear carbon fixation pathways have been surveyed, such as the CBB Cycle, Reductive Glycine (rGly) Pathway, THETA Cycle, Serine Cycle, RuMP Cycle, and more7.

There are tradeoffs among the cell-free pathways, such as the energy efficiency, the number of cofactors that must be regenerated, and the number of enzymes involved, but they all face the same core challenges. Any cell-free carbohydrate pathway probably requires several dozen enzymes, as well as an ATP-regeneration system to drive the reaction/cycle forward. Furthermore, enzyme manufacturing is still relatively expensive, and enzymes usually require immobilization or protein engineering to improve their useful lifetime.
Fats
Thermochemical Pathways
The best looking option for synthetic fats might be a chemical process, not a biological one! There is no reason you need biology to produce fats; they’re fairly simple molecules consisting of hydrocarbon chains. The petrochemical industry has spent decades figuring out how to synthesize and do useful things with hydrocarbons at industrial scales.
One company pursuing a chemical route to fats is called Savor. Their thermochemical process converts renewable energy into edible fats, using either H2/CO2 or CH4 as the feedstock. Transparently, I don’t know much at all about their process. Claude and I suspect that they could start with methane and use a pathway like: Methane Feedstock → Steam Methane Reforming → Fischer-Tropsch Synthesis → Oxidation to Fatty Acids.
This blog post from Synthesis (a VC fund invested in Savor) lays out the many advantages of their approach: it can be precisely tuned to produce a range of fat products, it’s based on mature chemical processes (less scale-up risk), and has a path to price parity with commodity oils (they claim).

I wanted to do a rough check on the techno-economic feasibility of synthetic fat production. I crudely approximate capital costs using a reference methane-to-fet-fuel facility and assume a 50% higher CAPEX due to food grade requirements and the additional processing steps needed to oxidize the fat. I also assume a WACC of 10%, and a profit margin of 10%.
The results shown above indicate that cost-competitive thermochemical fats are within reach of palm oil, the cheapest commodity food oil in the world. While only a few squares in the chart reach the $0.80/kg palm oil benchmark, it’s worth noting that commodity prices have spiked as high as $1.60/kg in 2022. If the palm oil supply chain was significantly disrupted, nearly all squares would be cost competitive!
I think a Savor-like thermochemical process could be the future of sustainable fat production, but hinges on significant cost reductions for green methane, (or equivalent amounts of green hydrogen and captured CO₂. I imagine that CPG companies would be willing to pay a premium for a palm oil substitute with less price volatility and a significantly lower carbon footprint, hence my optimism.
Benefits of synthetic food
Why should we photosynthesize some foods in solar-powered bioreactors, when plants accomplish this feat already?
First, we could decouple farming from arable land. Plants require soil and a relatively narrow range of climates to grow, but synthetic food could be grown out in the middle of the desert, where there are no competing uses for land. Places like Nevada or Saudi Arabia could become the new breadbaskets of the world. Small islands like Singapore could have domestic food security.

Second, synthetic food would be robust to droughts, natural disasters, swarms of cicadas, etc. The biggest tail risk to worry about would be a sun-blocking event, but in that scenario we could swap out solar for another electricity source. The fungibility of electricity is a key advantage. In a disaster scenario, you could imagine France turning nuclear energy into food, or Iceland turning geothermal energy into food.
Third, synthetic food production could be much less land-intensive. As we saw above, solar-powered biomass fermentation could produce 10x more protein per unit area, and 2x more calories than soybeans. I expect that thermochemical fats could also be an order of magnitude more land-efficient than oil crops.
Finally, synthetic food is programmable. In principle, biomanufacturing could produce any molecule we want, including foods, chemicals, or materials that haven't been made before. This is a big paradigm shift, from discovery and domestication to engineering and design.
I'll temper my techno-optimism by saying that it doesn't make sense to produce all, or even most, of our food supply in bioreactors. Biomanufacturing facilities are expensive to build, whereas plants are self-assembling factories with near-zero CapEx. It will be a while, in my view, for synthetic food to compete on price with commodity crops like soy, wheat, rice, maize, etc.
Looking ahead
By now, I hope to have convinced you that synthetic food is at least worthy of more exploration. Powered by renewable energy, a synthetic food supply could reduce land use and emissions while hedging against natural disasters. It could drastically improve food security in places where arable land is scarce.
Electricity is already abundant enough to feed the world with synthetic food many times over, though that electricity isn’t clean enough yet. We produced 22 MWh of energy per person in 2022, while the food-energy needs of a person are on the order of 1-2 MWh per year8.
The true challenge for synthetic food is not energy, but cost. Economic viability depends on significant cost reductions in biomanufacturing infrastructure, renewables, and the production of next-generation feedstocks like green methane, methanol, and formate.
The 0.75 m2 figure is based on calculations in this paper's introduction, and is the lower bound for how much land you need to feed a person if they could somehow absorb sunlight with 100% efficiency. Total land use for agriculture is 48M km2, and there are about 8.1B people, coming out to ~6,000 m2 per person.
Of course, phasing out animal agriculture would free up a massive amount of land, as I explained in the previous post. Land scarcity is driven more by what we eat than how much we eat.
Our World in Data puts cumulative global emissions around ~1,700 gigatons CO₂.
Rewilding cropland would sequester a lot of carbon too; livestock isn't the only culprit. I think livestock should be the focus of land restoration because (1) they cover the largest fraction of agricultural land, and (2) they are a much less productive form of food in terms of kcal/hectare.
A nitrogen source, like ammonia, is also required for fermentation. However, I'm going to sidestep the problem of sourcing green ammonia for now.
Even if you did draw power from the grid, the cheapest electricity prices are also intermittent. So you'd still want to be able to ramp feedstock production up and down, which also creates a need for temporary feedstock storage.
I would be remiss if I did not acknowledge how much of the existing research on synthetic carbon fixation pathways was either inspired or done by Arren Bar-Even.
To be generous, 3,000 kcal/day * 365 days/year * 1.162 Wh / kcal = 1.27 MWh. If you could eat electricity, your food would cost a few hundred dollars per year.




D-glucose isn't the only metabolizable sugar in formose. Among the D sugars there's glyceraldehyde, ribose, xylose, ribulose, xylulose, mannose, galactose, fructose, tagatose, and sedoheptulose, plus possibly allose, altrose, and talose. Among the L sugars there's glyceraldehyde, xylose, xylulose, gulose, galactose (extremely poorly though), psicose, and sorbose (though it's also a toxic compound), plus possibly allose, altrose, idose, and talose. Dihydroxyacetone can also be metabolized, as can glycerol, glyceric acid, allitol, DL-altritol, D-sorbitol, D-mannitol, galactitol (very poorly), D-gluconic acid, L-gulonic acid, D-galactonic acid, L-iditol, and possibly ribitol, DL-arabinitol, L-galactonic acid, and L-idonic acid. The other aldoses can also potentially contribute energy by way of aldehyde dehydrogenase, but they could also sap antioxidant defenses via aldose reductase. Another way would be via bacterial fermentation in the gut (which generates short-chain fatty acids that can be absorbed). Branched-chain sugars would be the most troublesome of the bunch, as only D-apiose and D-hamamelose are known to be metabolized by bacteria or any other organism. As for formose as a whole, studies have shown that amounts above 20% of the diet killed subject animals (very possibly through a combination of severe diarrhea, antioxidant depletion, osmotic stress, glycation stress, high anion gap acidosis, and phosphate trapping), with purified formose enabling longer survival compared to crude formose, and amounts above 10% impaired weight gain.
An addendum: under some conditions formose can include C9 sugars, and some of formose's toxicity could be caused by non-metabolizable C7, C8, and C9 sugars; D-mannoheptulose is known to inhibit glycolysis and insulin secretion.